Two Advocate Aurora Health hospitals in Illinois and Wisconsin have joined a clinical trial evaluating a device implanted in the heart that is intended to reduce the risk of stroke in patients with atrial fibrillation, or AFib.
The international clinical trial, known as CHAMPION-AF, will directly compare a procedure called left atrial appendage (LAA) closure using the WATCHMAN FLX™ device to the current standard treatment of non-vitamin K oral anticoagulants in patients with non-valvular AFib.
“The current standard of care, life-long use of blood-thinning medications to reduce the risk of stroke, can bring a risk of serious bleeding,” said electrophysiologist Jasbir Sra, MD, Advocate Aurora Research Institute’s site principal investigator for Aurora St. Luke’s Medical Center in Milwaukee, the only site in Wisconsin participating in the study. “The WATCHMAN FLX™ device is currently used as a treatment alternative in patients who can tolerate oral anticoagulants but are considered poor candidates for their long-term use. We’re pleased to participate in the CHAMPION-AF clinical trial to evaluate the next-generation WATCHMAN FLX™ left atrial appendage closure technology as a first-line therapy for stroke-risk reduction in patients with non-valvular AFib, including those at low-to-moderate bleeding risk.”
AFib is the most common type of heart arrythmia, affecting 2.7 million Americans, and patients with AFib are five times more likely to suffer a stroke than someone with a normal heart rhythm, according to the American Heart Association.
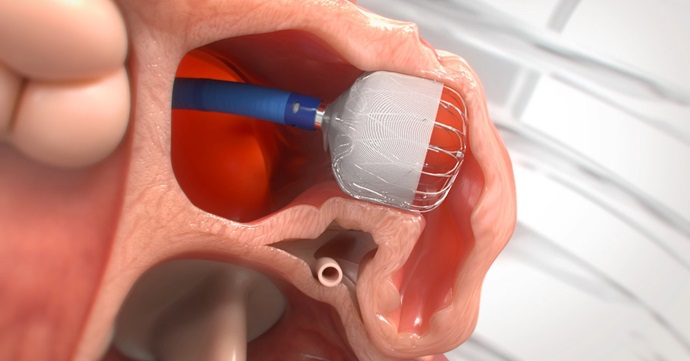
“The irregular heartbeat caused by AFib can lead to the stasis of blood in the LAA, which is a small, blind-ended sac in the wall of the heart,” said electrophysiologist William Spear, MD, the Research Institute’s site principal investigator for Advocate Christ Medical Center in Oak Lawn, Illinois. “Prior studies have shown that, in patients with non-valvular AFib, more than 90% of stroke-causing blood clots that come from the heart are formed within the LAA, and by closing the LAA with the WATCHMAN FLX™ device, we are able to reduce the risk of stroke without the need for long-term oral anticoagulation.”
The WATCHMAN FLX™ device is designed to permanently close the LAA, which stops blood from entering and pooling inside and lowers the risk of blood clots and stroke in these patients.
Researchers plan to enroll approximately 3,000 participants, who will randomly receive treatment with either commercially available non-vitamin K oral anticoagulants or the WATCHMAN FLX™ device. Study researchers will continue follow up of participants for five years.
The study, “CHAMPION-AF Clinical Trial (CHAMPION-AF),” is sponsored by Boston Scientific, manufacturer of the WATCHMAN FLX™ device.
To learn more about Advocate Aurora’s research, visit aurora.org/research.
About Advocate Aurora Research Institute
Advocate Aurora Research Institute is a not-for-profit, limited liability company of Advocate Aurora Health. Advocate Aurora has emerged as a national destination for patient-centered bench, translational and clinical research, and the Research Institute unifies the innovative research efforts throughout the health system. Advocate Aurora researchers focus on rapidly translating new discoveries from the scientist’s bench to the patient’s bedside and into the community we serve to improve options and outcomes that change not only the lives of individuals, but transform the health of populations.